Cancer Case Reporting in California
In support of physician cancer case reporting for Meaningful Use (MU), the California Cancer Registry (CCR) is working with the California Department of Public Health (CDPH) Health Information Exchange (HIE) Gateway with implementing electronic cancer case reporting. Successful ongoing submission of electronic health records (EHR) to the HIE Gateway will assist eligible participants (EP) in meeting the MU requirements.
For MU Stage 3, cancer case reporting falls under the Public Health Registry Reporting measure.
- Meaningful Use
- Onboarding Process
- Reporting Requirements
- FAQ
- Contacts
- Resources
Meaningful Use - Cancer Reporting in California
The California Department of Public Health (CDPH) is coordinating all reporting for Meaningful Use (MU) for California. CDPH is utilizing a Health Information Exchange (HIE) Gateway to receive and process MU files. For more information on the CDPH HIE Gateway, please go to Health Information Exchange Gateway website
To participate in MU for Cancer Reporting, Physician Offices (PO) must meet the HL7 Clinical Document Architecture (CDA) 2.0 standard, and messages must conform to the CDA implementation specifications found in the Implementation Guide for Ambulatory Healthcare Provider Reporting to Central Cancer Registries. In addition, the technology used by the PO to generate the HL7 CDA .XML message has to be a Federally Certified 2014 Edition EHR technology.
There are two transmission options for submitting MU data for Cancer Reporting:
If you do not know your transport method, please ask your Information Technology representative or your Electronic Health Record vendor prior to enrolling to submit MU cancer data.
Meaningful Use Stage 3
The California Cancer Registry is declaring its readiness to meet the Stage 3 Meaningful Use Cancer Measure. CCR will be able to meet the 2015 Edition CEHRT criteria, including incorporation of the updated Cancer Implementation Guide HL7 CDA ® Release 2 Implementation Guide: Reporting to Public Health Cancer Registries from Ambulatory Healthcare Providers, Release 1, DSTU Release 1.1 – US Realm
Instructions for Enrolling in MU Cancer Reporting
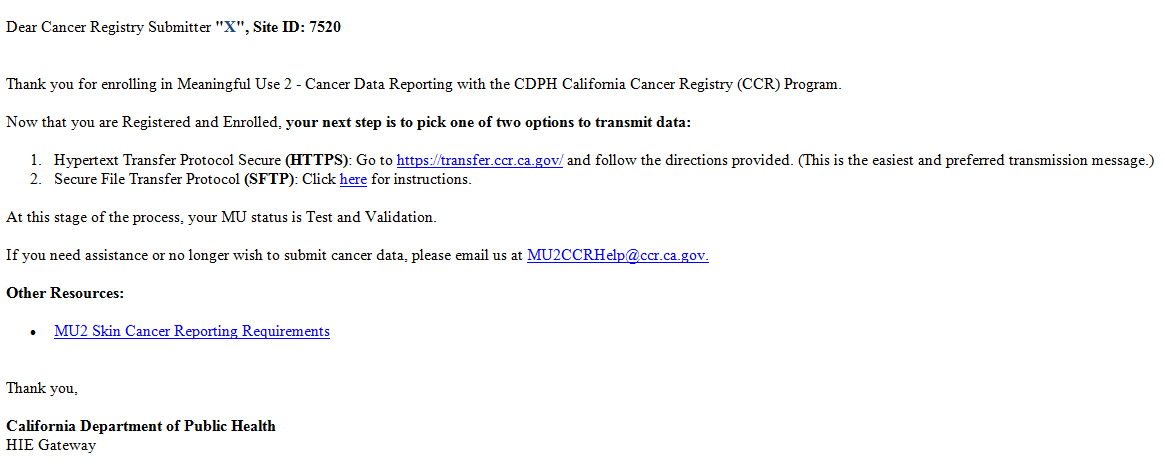
- Open the following URL in your web browser:Health Information Exchange Gateway Account Registration
- Follow the on-screen directions to Create a New Account and Click Create Account.
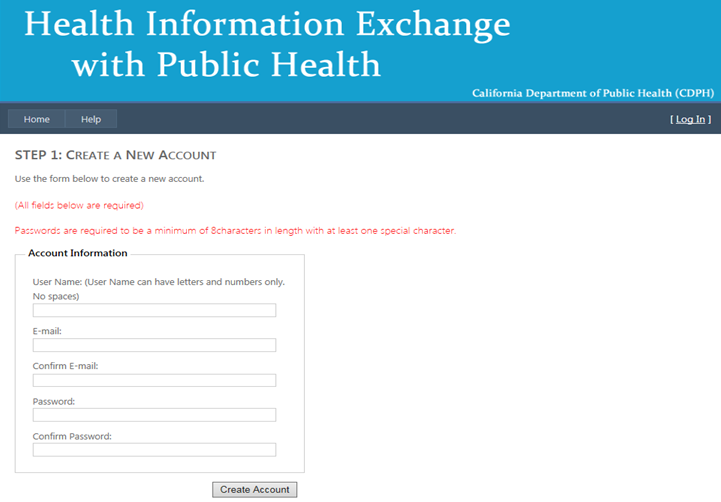
- You will receive an email from HIEHelp@cdph.ca.gov, similar to the image below, requesting confirmation of your registration.
- Click on the link in the email to confirm your registration.
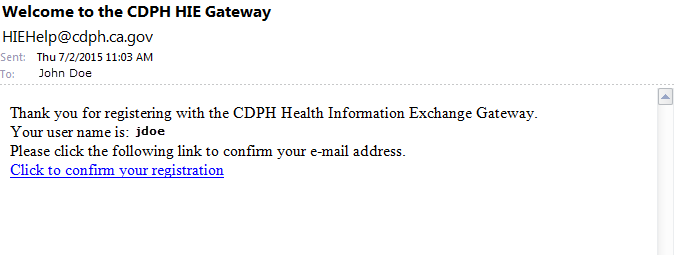
- HIE account activation page is displayed. Click on the link provided.
- You will be redirected to the Log In page.
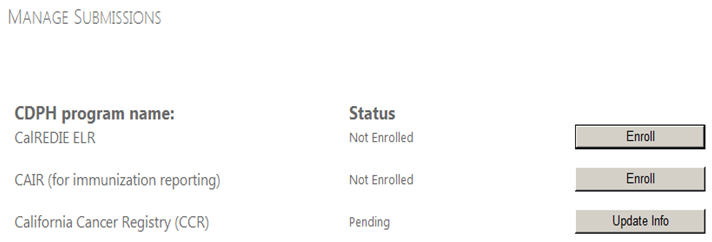
- The screen below is then displayed:
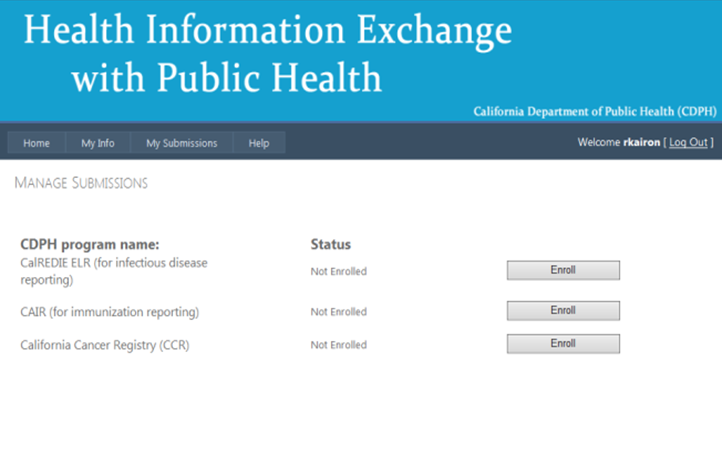
- On the Manage Submissions screen, Click Enroll for the California Cancer Registry.
- Next, you will receive an email from CDPH HIE Gateway, letting you know that you have successfully enrolled with CCR and providing you directions and options for transmitting your data.
A "Thank you" screen will appear that references the email mentioned below.
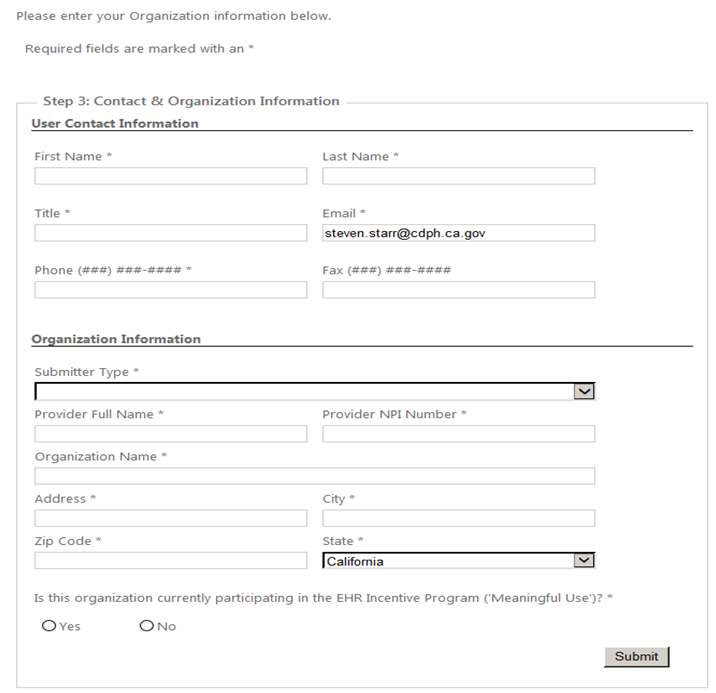
At a minimum, please complete the required fields marked with an asterisk and then Click Submit.
You are then directed to the Manage Submissions page:
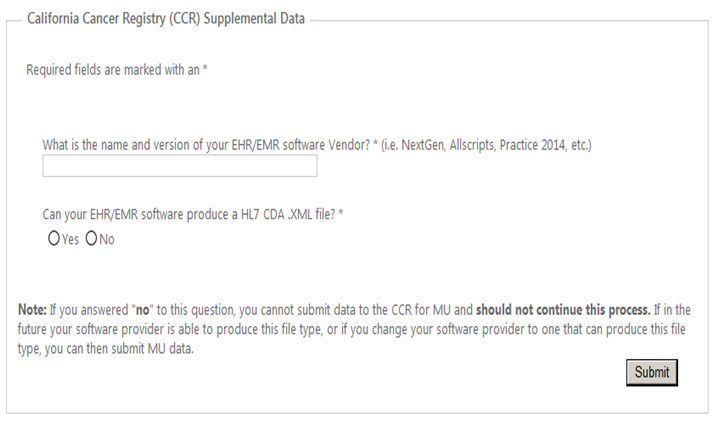
You are then directed to the CCR Supplemental Data page. Answer the questions and Click Submit.
The following screen appears:
Testing/Validation
Now that you are Registered and Enrolled with the CDPH HIE Gateway, you are ready to make the decision how you want to submit your MU cancer data. There are two options available to you that will allow the data to go directly to the CCR;
- Hypertext Transfer Protocol Secure (HTTPS): Go to California Cancer Registry Hypertext Transfer Protocol and follow the directions provided. (This is the easiest and preferred transmission message.)
- Secure File Transfer Protocol (SFTP): Go to California Cancer Registry Secure File Transfer Protocol and follow the directions provided.
Once you have selected your transmission option, you are able to submit HL7 CDA .XML files to the Cancer Registry. For the Testing & Validation phase, you are asked to submit 5 test messages that pass the CDC Validation process. Once your office has achieved this, you will then be moved to Production for ongoing data submission.
Ongoing Data Submission
You can begin ongoing data submission in compliance with MU for Cancer Reporting.While actively engaged in the steps above, you will be receiving emails and possibly telephone calls from CDPH HIE and CCR. You should maintain copies of these communications for EHR Incentive Program audit purposes.
If you have any questions regarding these processes, you can contact our office at this email address: MU2CCRHelp@ccr.ca.gov
MU2 Skin Cancer Reporting Requirements
Skin CancersBelow are the MU requirements for skin cancers. This information is in California’s Volume I., Section II.1.4 – II.1.4.1-II.1.4.2
Reportable skin cancers include:
- Skin cancers in the genital sites (any histology) (vagina (C52.9); clitoris (C51.2); labium (C51.0); vulva (C51.9); prepuce (C60.0); penis (C60.9) and scrotum (C63.2) are reportable
- Malignant tumours of the skin such as
- adnexal carcinomas (e.g., carcinomas of the sweat gland,
- sebaceous gland,
- ceruminous gland, and hair follicle,
- adenocarcinomas,
- lymphomas,
- melanomas including the following:
- Early melanoma insitu*
- Evolving melanoma insitu*
- sarcomas,
- Merkel cell tumors,
- Any carcinoma arising in a hemorrhoid is reportable since hemorrhoids arise in mucosa,
not in the skin.
None-Reportable to the Cancer Registry:
- Basal cell carcinomas of the skin
- Epithelial carcinomas of the skin
- Papillary carcinomas of the skin
- Squamous cell carcinomas of the skin
- Early Melanoma
- Evolving Melanoma
Note: The term “insitu” must be included in diagnosis in order for early and evolving melanoma insitu to be reportable.
CCR Reportability Guide
The link below is the reportability guide for certain histologies and sites for tumors that are reportable or not reportable to the CCR. https://www.ccrcal.org/download/82/site-pdf-links/3756/vol-iv-2016.pdfClick on Part I.1.6.7
HIE Gateway Frequently Asked Questions
If you have any more questions specific to the Public Health-related Meaningful Use objectives, please send your inquiries to MeaningfulUse@cdph.ca.gov. For general questions related to Meaningful Use and the CMS EHR Incentive Program, please contact CMS or the Department of Healthcare Services. If you have technical questions or problems related to the CDPH HIE Gateway, please send to HIEhelp@cdph.ca.gov. if you have any more questions related to Cancer Case reporting, Please email us to MU2CCRHELP@ccr.ca.gov.

What is Meaningful Use?
What are the benefits of Electronic Health Records (EHR)?
How does EHR benefit the cancer registries?
What are the specifications for electronic reporting from ambulatory healthcare providers’ EHR systems to public health central cancer registries?
Will registries need to develop a local specification for the Health Level Seven (HL7) Clinical Document Architecture (CDA) physician reporting document?
Is a timing element described in the cancer reporting process as to when data exchange will occur?
What’s the difference between a “software vendor”, and the “software name and version”?
What are the goals of the meaningful use?
Where can I find more information on meaningful use?
What is a cancer registry?
How does the California Cancer Registry (CCR) get information on cancer cases?
What data does the CCR collect?
What are the data used for?
What does the CCR do?
Is the information kept confidential?
Do I need to register at the CDPH HIE Gateway?
If you intend to submit public health data to a state program, you may need to register at the Gateway. Many providers, professionals, and organizations that are required by law or regulation to submit specific health data to public health agencies will need to register. Other Organizations/Sites will need to register in order to submit public health data to qualify for 'Meaningful Use' incentive payments. Types of public health reporting currently available through the Gateway include:
- Laboratory results reportable to CalREDIE
- Immunization data reporting to CAIR (NorCal, Gr. Sac, Bay, Central Valley, Central Coast, LA-Orange, and Inland Empire regions only)
- Cancer cases from ambulatory healthcare providers can be reported to the cancer case registry
- Blood lead laboratory test results reportable to CDPH Childhood Lead Poisoning Branch... Coming Soon!
I can't log in. The user name and password I chose do not work.
Please review the following helpful tips to troubleshoot log in issues.
- Verify that you have clicked the email confirmation link that was sent to you.
- Check to make sure your user name matches what is listed as user name on your confirmation email.
- Your password is at least 8 characters in length and includes a special character (e.g. @, #, $, etc.).
- Check to see if CAPS lock is on; passwords and user names are case sensitive.
- Try closing your browser, returning to hie.cdph.ca.gov and attempting to log in one more time.
What is Meaningful Use?
Meaningful Use is a set of standards defined by the Centers for Medicare and Medicaid Services (CMS) Incentive programs that govern the use of electronic health records that allow eligible providers and hospitals to earn incentive payments by meeting specific criteria. The goal of meaningful use is to promote the spread of electronic health records to improve health care in the United States.
Legislation: The American Recovery Reinvestment Act (ARRA) and Health Information Technology for Economic and Clinical Health Act (HITECH) in the stimulus law were signed on February 17, 2009, by President Obama and provide $19.2 billion in Health Information Technology (HIT) spending. ARRA HITECH legislation provides that eligible professionals and hospitals who demonstrate “meaningful use” of certified electronic health record technology are eligible for incentive payments. The adoption of EHR and HIT in high priority areas such as electronic prescribing, interoperable electronic health records and quality measure reporting can improve patient safety and the quality of healthcare.
What are the benefits of Electronic Health Records (EHR)?
Complete and accurate information: With electronic health records, providers have the information they need to provide the best possible care. Providers will know about their patients and their health history before they walk into the examination room.
Better access to information: Electronic health records facilitate greater access to the information providers need to diagnose health problems earlier and improve the health outcomes of their patients. Electronic health records also allow information to be shared more easily among doctors’ offices, hospitals and across health systems, leading to better coordination of care.
Patient empowerment: Electronic health records will help empower patients to take a more active role in their health and in the health of their families. Patients can receive electronic copies of their medical records and share their health information securely over the Internet with their families.
How does EHR benefit the cancer registries?
Population-based public health central cancer registries across the United States and most of Canada are mandated to collect complete and timely cancer diagnostic, treatment and outcome data from hospitals, physicians’ offices, treatment centers, clinics, laboratories and other sources. Recent shifts in cancer treatment away from hospital settings and towards ambulatory (non-hospital) healthcare settings are increasing the importance of ambulatory healthcare providers’ data for cancer surveillance. As ambulatory healthcare providers adopt modern EHR systems, the opportunity to automate cancer registry reporting from ambulatory healthcare provider settings is also increasing and becoming more feasible.
Here are some of the benefits of EHR::
- Improved accuracy and completeness of cancer surveillance data impact all areas of public health interventions.
- Data also provide baseline measures and performance measures for cancer-related interventions designed to reduce cancer incidence or improve early detection.
- Identification of disparities among various population subgroups, in stage at diagnosis or in treatment received, can inform interventions to reduce the cancer morbidity and mortality on disadvantaged populations.
What are the specifications for electronic reporting from ambulatory healthcare providers’ EHR systems to public health central cancer registries?
The standard used in electronic reporting from ambulatory healthcare providers’ office to central cancer registry is Health Level Seven (HL7) Clinical Document Architecture (CDA). This document is designed to guide EHR vendors and public health central cancer registries in the implementation of standardized electronic reporting.
An implementation guide for ambulatory healthcare provider was developed through a collaborative effort of the Centers for Disease Control and Prevention (CDC), National Cancer Institute (NCI) Surveillance, Epidemiology, and End–Results (SEER) Program, public health central cancer registries, EHR vendors, and the North American Association of Central Cancer Registries (NAACCR).
The implementation guide can be found in the following link: Implementation Guide for Ambulatory Healthcare Provider Reporting to Central Cancer Registries.
Will registries need to develop a local specification for the Health Level Seven (HL7) Clinical Document Architecture (CDA) physician reporting document?
State cancer registries do not need to develop local specifications. They should use the standard adopted by the Office of the National Coordinator for Health Information Technology (ONC), the Implementation Guide for Ambulatory Healthcare Provider Reporting to Central Cancer Registries [PDF-1.9MB], for eligible professionals (EPs) to report cancer data. If your state has requirements that are not included in this standard, please work with CDC to standardize these elements. Please be aware that EHR vendors will not be required to include these additional data elements. CDC will work with state cancer registries to identify any additional data elements that were not included for MU Stage 2. CDC plans to update the national specification to include consistent data elements in the MU Stage 3 standard.
Is a timing element described in the cancer reporting process as to when data exchange will occur?
The Implementation Guide for Ambulatory Healthcare Provider Reporting to Central Cancer Registries (commonly called the Cancer Implementation Guide) requires a report to be sent every time the EHR identifies an encounter with cancer as the first-listed diagnosis.
What’s the difference between a “software vendor”, and the “software name and version”?
- An example of a software vendor: Microsoft
- An example of a software name and version: Windows 7, or Internet Explorer 8.0
What are the goals of the meaningful use?
- Improve quality, safety, efficiency and reduce health disparities
- Engage patients and families in their health care
- Improve care coordination
- Improve population and public health
- Maintain privacy and security
Where can I find more information on meaningful use?
What is a Cancer Registry?
A cancer registry is a system for collecting, storing, and studying data on persons with cancer. Cancer registries are needed to measure the burden of cancer in our communities, to help identify the causes of cancers, and to find cures for these diseases.
California has a comprehensive cancer registry that covers the entire state. It is considered one of the best cancer registries in the world. State law requires that every cancer diagnosed in California be reported to the California Department of Public Health (CDPH) which manages the California Cancer Registry (CCR).
How does the California Cancer Registry (CCR) get information on cancer cases?
The law requires doctors, hospitals, and other facilities that diagnose and treat cancer patients to report information on cancer cases to the CCR. The CCR and regional registries work with local reporting facilities to ensure that information on cancer cases is complete and accurate.
The CCR collates all the data, performs additional quality control, and analyzes the data on a statewide basis. At each step of the process, strict procedures are in place to protect patient confidentiality.
What data does the CCR collect?
All data collected by the CCR are obtained directly from cancer patients’ medical records and include demographic, diagnostic, and treatment information on individual cancer cases.
- Demographic data include: patient’s name, address at time of diagnosis, sex, race, and age at diagnosis.
- Diagnostic data include: type of cancer (such as breast cancer) and stage of disease at time of diagnosis.
- Treatment data include: whether the patient had surgery, radiation, or chemotherapy as the first course of treatment.
The CCR does not interview patients.
What are the data used for?
CCR data are used to:
- Monitor the number of new cancer cases and cancer deaths over time;
- Examine disparities in cancer risk, treatment and survival;
- Examine treatment choices and other predictors of survival;
- Measure the success of cancer screening programs;
- Respond to public concerns and questions about cancer; and
- Conduct research to find the causes and cures of cancer.
Researchers have used CCR data to:
- Analyse geographic, racial/ethnic, and occupational differences in cancer risk;
- Evaluate the quality of medical care received by cancer patients; and
- Examine patient survival with respect to cancer type, extent of the disease, demographic characteristics, and other important factors.
What does the CCR do?
The mission of the CCR is to serve the public by collecting state-wide data, conducting surveillance and research into the causes, controls, and cures of cancer and communicating results to the public.
The CCR monitors the occurrence of cancer among Californians, both incidence (new diagnoses) and mortality (deaths). The CCR, which is operated by the CDPH and ten regional cancer registries, is an essential tool for the prevention and control of cancer in California.
By law (Health and Safety Code, Section 103885), all new cancer cases diagnosed in California residents since January of 1988 have been reported to the CCR, with strict guidelines to maintain patient confidentiality.
Is the information kept confidential?
Absolutely, the CCR was established in 1985 to serve as a key resource in the state for research into the causes and cures of cancer. It has a productive record of using CCR data for research and program evaluation to improve the spectrum of cancer control in California, including prevention, diagnosis, treatment and quality of life. CCR has very stringent policies and procedures to ensure that cancer data reported are maintained with the highest degree of confidentiality and privacy.
Cancer researchers must go through a rigorous process to access any CCR data. The CCR will only release patient contact information to qualified researchers under tightly controlled circumstances where the research has first been approved by the California State Committee for the Protection of Human Subjects (CPHS) Institutional Review Board. Research proposals are evaluated by CPHS to ensure patients’ rights are protected and the research justified. Additionally, a federally approved Institutional Review Board (IRB) at the researcher’s institution must also approve the research proposal. This IRB will also ensure that patient rights are monitored and protected.
Questions?
HIE Gateway Help
- Phone: (855) 281-4996
- Email: HIEHelp@cdph.ca.gov
Cancer Registry Specific Support
- For general MU2 Cancer reporting questions, please email MU2CCRHELP@ccr.ca.gov
Resources
- Medi-Cal EHR Incentive Program
- Centers for Disease Control and Prevention (CDC): Meaningful Use Information
- HealthIT.gov
Office of the National Coordinator of Health IT (ONC) EHR Resources
Register
Request Help
Just call (855) 281-4996 for support
or e-mail us at HIEHelp@cdph.ca.gov


